Welcome to the Virtual MS Center!
Ask any question you want about Multiple Sclerosis and one of our experts will answer it as soon as possible.
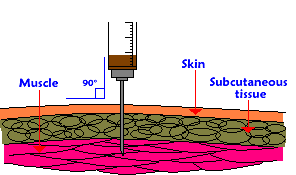 I will answer this question under the assumption that the "journey" asked about is regarding a disease modifying therapy (DMT) called interferon-beta 1a (also known as Avonex). This DMT is injected into the muscle once weekly and was approved by the FDA for the treatment of relapsing multiple sclerosis. Once injected (about 1 milliliter) into the muscle, absorption of the drug occurs and reaches the blood circulation which delivers it throughout the body. The active ingredient is a Type I interferon (which is identical to interferon made in our bodies as a response to a viral infection). The interferon is simply a string of protein building blocks (or amino acids) that also has a sugar chain attached to it. This complex (protein and sugar) interacts with receptors on various cells in our body (think of a lock and key relationship where the interferon is the key and the receptors are the locks). When the interferon unlocks the receptor on the surface of different cell types, it causes various chemical reactions inside the cell. One of these reactions includes the movement of a protein complex into the nucleus of our cells (this is the part of the cell that houses our DNA--or genetic code). This protein complex attaches itself to part of the machinery of the cell nucleus that expresses our DNA. (This machinery is like a Xerox copy machine of sorts. It recognizes specific genes and can literally copy the genes or translate the genetic code into other proteins that can leave the nucleus and do work that needs to be done throughout our body.) In the case of interferon, the machinery induces the expression of genes to fight viruses, provide anti-proliferative signals, and "modulate" the immune system. It is the induction of these "modulatory" genes that presumably exert the beneficial effects in MS patients (ultimately reducing the bad inflammation in the brain and spinal cord, the number of MS attacks, new scars on the MRI scans, and reduce the accumulation of physical disability over time). Clinical trial evidence suggests that the intramuscular (IM) route of administration was superior to intravenous (IV) but comparable to subcutaneous (just under the skin) in the sense that IM and subcutaneous injection reached a peak of biological effect at 48 hours rather than 24 hours by the IV route). Also the overall exposure (what we term the "area under the curve" when we plot blood levels of biological response markers over time after the injection) to the interferon was greatest using the IM route compared to IV or subcutaneous injections. Interferon is metabolized (ie, digested) by the liver as many drugs are, and eliminated from the body primarily through our kidneys. The half-life (the time it takes to half the amount of drug in the blood stream) is approximately 19 hours. A. Scott Nielsen MD MMSC Virgina Mason Multiple Sclerosis Center Comments are closed.
|
PLEASE NOTE: This information/opinions on this site should be used as an information source only. This information does not create any patient-HCP relationship, and should not be used as a substitute for professional diagnosis and treatment. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition.
Archives
June 2024
Categories
All
|