|
Whether or not one should eventually stop a disease modifying therapy (DMT) at some point in their journey with Multiple Sclerosis (MS) is a complex and, at times, emotional decision. Often people face this decision after placing their hopes on DMTs for well over a decade. There is some data to help with these decisions and several larger studies underway to provide even better data. Here is what the data suggests so far:
Revere (Rip) Kinkel MD Professor of Clinical Neurosciences Director of the Multiple Sclerosis Program Clinical Neurosciences Director University of California San Diego “Kids are not young adults”. Perhaps you’ve heard this quote before in another context. It seems obvious to most of us from everyday experience. In medicine there are special hospitals and separate medical specialties just for kids. You would not send your child to a cardiologist, who by lack of the appropriate modifier is assumed to be an adult cardiologist; instead, we have pediatric cardiologists and other kid specialists in fields like gastroenterology, pulmonology and neurology to name just a few.
The field of Multiple Sclerosis (MS) specialists is no exception. In 2006 the Network of Pediatric MS Centers was established through a National MS Society initiative and has subsequently expanded with further support from the NIH and the Guthrie Jackson Foundation. The initial focus of the pediatric MS network was to facilitate the appropriate diagnosis and comprehensive care of people under the age of 18 with MS and related diseases. This focus was justified since fewer than 5 % of people with MS experience an onset of the disease before the age of 18, misdiagnosis was commonplace and the consequences of misdiagnosis with a treatable condition like MS were large. The Pediatric MS network established separate diagnostic criteria for kids with MS allowing for earlier diagnosis and treatment, and an enhanced understanding of the age dependent clinical features of MS. We learned that children with MS often experience a very aggressive onset, sometimes with altered mental state and seizures, followed by a high rate of relapses, but we also learned that children can recover more completely than adults in the early stages of the disease with a longer interval between symptom onset and the development of progressive disease. In fact, unlike adults, it is extremely uncommon for kids with MS to experience progressive disease from the onset of the disease. The Pediatric MS Network also made it possible to organize clinical trials to determine the safety and effectiveness of disease modifying therapies (DMTs) in kids with MS. This was important since all clinical trials undertaken to obtain regulatory approval of MS DMTs in the US specifically excluded, and still exclude, children under the age of 18. Because of this age dependent exclusion the most effective DMTs, beginning with the approval of Tysabri in November 2004, were avoided in kids because of the lack of a specific FDA approval and the unknown risks in this vulnerable population. Without the evidence to support the safety of highly active disease modifying therapies, most kids with MS were relegated to treatment with potentially less effective and less tolerated injectable DMTs. There were, of course, exceptions mostly in academic centers, but injectable therapies remained the mainstay of management despite evidence of poor adherence to these DMTs, particularly in kids. Novartis altered this landscape with the FDA approval of Gilenya on May 11, 2018 for children with MS 10 years of age and older. In many ways Gilenya is a natural treatment choice for kids with MS. First and foremost, it is a once a day pill with few immediate side effects to negatively impact adherence to the treatment schedule. Most of the concerns in adults treated with Gilenya, notably the cardiac risks and the reactivation of herpetic infections like shingles, are less of a concern in kids. Yet we also knew that Gilenya and other medications in this class of drugs could potentially have adverse effects in kids that could not be anticipated based on the experience in adults. Importantly, the study was designed to compare Gilenya to Avonex, one of the more popular DMTs used as initial treatment in kids with MS. We already knew based on many years of experience that Avonex and the other injectable DMTs were relatively safe and effective in kids with MS. What we really needed to know was whether Gilenya offered greater benefits than a popular injectable DMT with an acceptable risk profile and acceptance by kids. It turns out that Gilenya is a lot more effective than Avonex in kids; 86 % of kids treated with Gilenya in the study were relapse free compared to only 46 % of kids treated with Avonex. Overall, there was an 82 % reduction in relapse rates. For kids, who tend to experience multiple relapses a year that take them away from school for prolonged periods of time, this is a very meaningful outcome. We also learned there is at least one unique risk when prescribing Gilenya in kids that can be potentially mitigated through preventative strategies. Six of the kids (5.6 %) treated with Gilenya experienced seizures compared to only 1 of the kids (0.9 %) treated with Avonex. This tells us that Gilenya may not be the best DMT in kids with a history of seizures or a susceptibility to seizures, particularly those not taking effective anti-seizure medications. Now that Novartis has led the way with clinical trials of MS DMTs in kids with MS, other companies are following their lead with numerous clinical trials for kids with MS now in various stages of planning or recruitment. This will help us better understand the relative effectiveness and sequencing of MS DMTs in kids in the near future. But what about other vulnerable populations excluded from MS clinical trials, notably those over the age of 55? As many of you know, all MS clinical trials exclude people over the age of 55 from participation and the average participant in MS clinical trials is approximately 37 years old. This leaves MS specialists with virtually no information on the effectiveness and safety of DMTs in older, more vulnerable population. This is a huge problem since unlike pediatric MS, which is a rare condition, people between the ages of 55 and 64 now represent the peak prevalence age group for MS. In the next blog we will begin to address the issue of MS management in older MS patients, starting with the need for specific clinical trials for people over the age of 55. Stay tuned. Revere (Rip) Kinkel MD Professor of Clinical Neurosciences Director of the Multiple Sclerosis Program Clinical Neurosciences Director University of California San Diego Here is My Question:
I was diagnosed with MS 7 years ago. I have been on Tysabri for 3 years and haven’t had any major side affects. I’ve just had blood tests done and my white blood cells are high, he thinks it could be from the Tysabri but also indicated if not from Tysabri it could be leukemia. Should I contact my consultants at the Austin Hospital in Melbourne where I see my neurologist to discuss with them. I'm trying not to get overwhelmed. Answer: It is not uncommon to see elevated white blood counts in patients on Tysabri. The majority of the time, the total white blood count (WBC) will remain less than 15,000, but on occasion we see elevations as high as 20,000. This is a result of the normal mechanism of action of Tysabri and not harmful at all. The type of white blood cells seen on the blood smear include normal white blood cells and some precursor cells. The elevations in total WBC begin as early as a month after the first dose and tend to persist. Some cell types, for instance monocytes, may show continued elevations over the first year. If your WBC elevation has been present since the start of treatment, there is no cause of concern. If your WBC elevation is new or much higher than previously, then you should probably be evaluated by a hematologist. They specialize in disorders of blood cells. Leukemia will cause an elevation of both normal and abnormal blood cell types and is typically very easy to differentiate from the WBC elevations observed in people on Tysabri. If your doctor(s) has a question, I suggest they contact a local MS specialist or call the Biogen Medical Liaison Office. Let us know what you learn. Revere (Rip) Kinkel MD Professor of Clinical Neurosciences Director of the Multiple Sclerosis Program Clinical Neurosciences Director University of California San Diego Here is My Question:
For over 5 years, my mother has experienced progressive loss of leg strength and has an inability to walk with lots of fatigue of legs, heavy legs, jelly legs, weakness in legs, and numbness along with hip pain at times. She was diagnosed with possible OA in feet and slight L-spine herniation/bulge but stated not enough to cause issue. RA level was normal. She experiences tingling and numbness in fingers at times as well. No pain in legs just a pins and needles, weakness, jelly feeling that causes her to stumble. EMG/NCV,eye, DXA exams normal. She did have shingles earlier this year. She has to hold on to things when walking, showering, etc. Most issues are from hips down. She went to PT issues, but it helped very little. I'm worried this is MS that has been slowly progressing, but how can I get doctor's to test or look further into her symptoms without them all stating the same thing, that it is related to her SI/piriformis and OA from age? Thank You. Answer: MS is a clinical diagnosis (which is extensively documented on the site) which is best reviewed and applied by an MS specialist who is well versed in the McDonald Criteria. If you provide the city/state of your family, we may be able to give you some names of a specialist that you could reach out to for another opinion. A. Scott Nielsen MD MMSc Neurologist and MS Specialist at Kaiser Permanente The concepts of progression and inflammation are somewhat independent of one another.
When considering inflammation in MS, we are referring to confirmed new relapses of neurological disability that is due to a new discrete inflammatory event in the central nervous system. Another sign of inflammation is new or enlarging, or contrast enhancing lesions on MRI. Evidence of inflammation such as these examples suggest that the disease course would be benefitted by a disease modifying therapy. It is possible to show signs of inflammation but also to show signs of disability progression. This is typically defined as the accumulation of permanent disability that can be detected on your neurological examination. This can be reflected in many ways. More typically, ambulatory dysfunction is readily seen as a sign of progression. However, progression can also occur in coordination problems, bladder or bowel changes, cognitive dysfunction, visual disturbance, etc. The point is that the worsening in the exam doesn’t get better but remains 3 or even 6 months later on the exam (when we see this we call it “confirmed” progression). The progressive phase of MS, which is experienced by the majority of MS patients later on in the disease course, is felt to be a different phase of the disease and not entirely explained by inflammation (indeed, the progressive phase often occurs while there is no signs of ongoing inflammation). In other cases, you can see both in the same patient. When this happens, some of our MS disease modifying therapies may be able to slow down the disease course. The b-cell depleting therapies (such as Ocrevus or off-label dosing of Rituxan) have data showing they can slow down the progressive phase in these types of patients. The important take away from this is that we are less interested in using those old names for MS (ie, relapsing-remitting, secondary progressive, primary progressive, etc), but we are more interested in knowing if there is ongoing signs of inflammation which would argue for use of an appropriate Disease Modifying Therapy that may help slow down the disease course which should translate into more time with a better quality of life (compared to doing nothing to treat the disease). A. Scott Nielsen MD MMSc Neurologist and MS Specialist at Kaiser Permanente I have never seen a drug prescribed to so many MS patients over such a short period of time, as I have witnessed with Tecfidera since its marketing release in March 2013. The reasons for the rapid embrace of this therapy by physicians and patients alike are all valid; this is a relatively safe, oral agent with efficacy at least as good as other oral agents and probably better than most injectable therapies (interferons and copaxone). But this massive prescribing creates one particular problem; both patients and physicians are using this drug without being fully aware of the potential side effects and how to effectively manage them. This can often lead patients to stop treatment prematurely. So I wanted to take some time in this blog to share my experience in helping patients and colleagues manage or prevent side effects from Tecfidera.
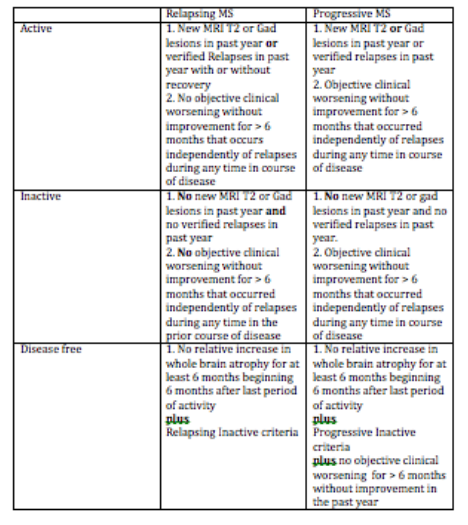
The most common side effect of Tecifidera is flushing. If you have ever taken nicotinic acid for high triglyceride levels you know what this means. About 30 minutes to 2 hours after a Tecfidera dose (longer if you take it with food), you may develop redness, warmth and a prickly sensation typically involving the face, chest and arms. This can last for 20 to 30 minutes before subsiding. Rarely, the flushing can be severe with associated symptoms like your heart racing (palpitations) and chest tightness. Some patients who were not informed of this potential side effect thought they were experiencing an allergic reaction. This side effect can be blocked or minimized (if even necessary to treat) by taking an aspirin (81 to 325 mg) in the morning. If you are unable to tolerate aspirin or allergic to aspirin you can try ibuprofen (eg motrin) or acetomenophen (eg. Tylenol) but they do not tend to work as well as aspirin. Less commonly, patients experience night sweating after starting Tecfidera. Many patients have found that an aspirin (81 to 325 mg) at bedtime can minimize this problem. The potentially more troublesome side effects of Tecfidera involve the gastrointestinal tract. These side effects can be separated into upper and lower GI symptoms. The upper GI symptoms include nausea and pain in the epigastric region (just below the sternum or breast plate), and are caused by Tecfidera sitting in the stomach too long. Taking Tecidera with food helps but is often not good enough. I have found that a drug called metoclopramide (5 to 10 mg) taken 1 hour before each dose of Tecfidera helps prevent the nausea and clears the drug more quickly from the stomach to prevent pain. If this symptom is a problem for you, make sure you ask your MS specialist about this remedy. Since most side effects begin when the doctor is not available, I always send patients home with a prescription for metoclopramide to have available if needed. Lower GI side effects, which include cramping and diarrhea, are equally troublesome. Thankfully, you usually do not need your doctor to help out; simply pick up some over the counter loperamide (the brand name is Imodium) and take 2 mg twice a day until this symptom subsides. If this does not work you may need to contact your MS specialist for a prescription strength medication. The remarkable thing about Tecfidera is that most of the side effects tend to either disappear or become insignificant after 4 to 8 weeks. It is during this period of time that you may need help managing these symptoms. If the side effects remain problematic for more than 2 months this may not be a good drug for you. When side effects are a problem, they usually disappear within 24 hours of stopping the drug. Certainly, side effects that persist or worsen more than 24 hours after stopping Tecfidera should prompt you to contact your doctor immediately. Often I will have patients experiencing annoying or troublesome side effects stop Tecfidera for a few days before restarting the medication at a lower dose and gradually increasing to full dose again using the strategies discussed above to prevent a return of the side effects. As always, every patient situation is different and you should discuss these issues with your doctor before initiating one of the remedies mentioned in this blog. NOTE: The medical information on this site is provided as an information resource only, and is not to be used or relied on for any diagnostic or treatment purposes. This information does not create any patient-physician relationship, and should not be used as a substitute for professional diagnosis and treatment. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. A Modern definition of MS Disease Types useful for Therapeutic decisions March 23, 2016 I am often asked how MS specialists characterize the disease course and use this information for making treatment decisions. Most of the patients I meet want to know if they have relapsing remitting, secondary progressive, primary progressive or progressive relapsing MS. These are old classification terms based only on clinical features and then to create confusion without helping us with management decisions. The table below shows a classification system that we developed initially during the CHAMPIONS study and refined over time as we developed better tools to monitor. MS activity with MR imaging. This classification eliminates the older terminology and concentrates on defining inflammatory activity as the primary driver of treatment decisions and cessation of disease progression and brain atrophy (both linked concepts) as the ultimate goal of treatment (disease free). This criterion does not incorporate known risk factors for disease activity and worsening which we use concurrently to determine the most appropriate disease modifying therapy. In other words not all relapsing active or progressive active patients are the same in terms of treatment decisions. For instance, those people with MS who experience severe or frequent relapses with incomplete recovery require treatment with highly active DMTs whereas those early in the course of the disease with mild infrequent relapses and a low MRI burden of disease do not require highly active DMTs. The newer classification begins by characterizing MS as Relapsing or Progressive. This is entirely determined by clinical features. The course is then qualified as active or inactive based on a combination of clinical and MRI features. Relapsing patients have periodic, often rare relapses, with little if any continuous worsening of their condition between relapses that can be measured. Relapsing patients also tend to experience new T2 or Gad-enhancing lesions even in the absence of clinical relapses. We now combine clinical relapses and new MRI lesions as our definition of inflammatory activity over a given interval of time. Progressive patients slowly worsen over periods in excess of 6 months in a way that we can measure in the clinic, although they often spontaneously experience relatively long periods of clinical stability even without treatment. Progressive patients may also experience relapses (see above for activity definition) and new MRI lesions, particularly in the early course of the disease. These progressive active patients are excellent candidates for disease modifying therapy (DMT), especially newer highly active DMTs. To be completely disease free you must meet the additional criteria of experiencing a rate of brain atrophy no greater than healthy controls following recovery from a period of activity. This is a high standard to achieve by treating people with most current DMTs and, as a result, most centers do not currently quantify brain volumes on their patients. This is rapidly changing and will be an important treatment goal in the future Symptoms of progressive disease can vary but tend to involve previous areas of involvement by relapses or MRI activity. For instance, those people who tend to experience weakness on the right side during relapses have a greater tendency to experience slowly worsening right sided weakness as a sign of progressive disease and those with a larger cerebral burden of disease with early cognitive difficulty often experience a decline in cognition as a sign of progressive disease. The actual symptoms vary tremendously from one patient to another. The time course for these stages in also highly variable; Relapsing disease tends to last for 5-25 years and progressive disease tends to begin 0 to 25 years after onset. As mentioned above we use various risk factors to determine a persons risk of experience more aggressive disease in the near future. These risk factors are exclusively clinical and MRI measures at present but will likely incorporate other biomarkers in the near future. We tend to use disease-modifying therapies (DMTs) in those patients meeting activity criteria, whether progressive or relapsing in type. Those with long standing progressive disease without any activity do not respond as well to any of our current therapies. Revere (Rip) Kinkel MD Director of the Multiple Sclerosis Program Professor of Clinical Neurosciences University of California San Diego
Here is My Question:
What MRI machine and protocol best detects any changes in lesions or enhancing lesions? I'm feeling physical changes, but the MRI report shows no changes and I want to be informed. Thank you so much for answering my question. Answer: An MRI done to detect a change in disease state is only as good as the last MRI scan your received. Otherwise you are trying to compare apples to oranges. Let me explain. Imagine you obtained the highest resolution MRI scan available with the thinnest possible slices and all your brain tissue is imaged (we call this a volumetric image acquisition). This scan may show 60 discrete lesions with a resolution allowing you to confidently identify lesions as small as 1 mm diameter in the cortex. This will not help you if the prior MRI was obtained on a conventional 1.5 or even a 3.0 Tesla scanner with slice thickness of 3 to 5 mm and a gap between slices. The conventional scan may only identify 20 lesions and any comparison of the scans may suggest, inappropriately, that you suddenly developed 40 new lesions !! Other technical factors can make comparisons difficult even when the scans are obtained on the same scanner. This is why we recommend all our patients obtain a new baseline study on a 3 Tesla MRI scanner using volumetric 3-dimensional imaging with pulse sequences allowing us to perform automated registration and segmentation for comparisons of scans between time points. Unfortunately, few centers have this capability at present. For the time being it is best for you to obtain repeat MRIs on the same scanner used previously using the same imaging protocol and slice orientation for all scans. Even when this is done, changes on MRI do not correlate one to one with changes in symptoms or changes examination. For over three decades, we have referred to this phenomenon as the clinical radiological paradox. In many ways MRI findings and changes on MRIs over short intervals (1-2 years) do a better job of predicting clinically meaningful changes in neurological function that may occur in the future Gadolinium enhancement on an MRI represents new breakdown of the blood brain barrier and is associated with acute inflammation. There is not a one to one relationship between relapses and gadolinium enhancement although enhancement may flare up around periods of relapses. This gadolinium enhancement does not correlate well with future disability in untreated patients but does correlate well with future lack of response to a disease modifying therapies if the enhancement persists on future scans at least 6 months after starting the disease modifying therapy. Highly active disease modifying therapies (such as Tysabri, Alemtuzumab, Rituximab and Ocrelizumab) virtually eliminate gadolinium enhancing lesions and are often not obtained after an individual is stable on a highly active disease modifying therapy for more than a year. The vast majority of enhancing lesions persist as T2 bright spots without gadolinium and will be detected as new interval lesions on a later scan. Revere (Rip) Kinkel MD Director of the Multiple Sclerosis Program Professor of Clinical Neurosciences University of California San Diego Roche Pharmaceuticals, the owner of Genentech, announced positive phase III study results of their anti-CD20 monoclonal antibody called Ocrelizumab yesterday. Ocrelizumab is a humanized monoclonal antibody targeting CD20 on mature B cells, whereas Rituximab is a Chimeric antibody (part mouse and part human) with the same target. This clears the way for FDA approval of Ocrelizumab by late 2015 or early 2016. The two phase III studies involved 1656 relapsing MS patients (both relapsing remitting and secondary progressive) randomized to either an Ocrelizumab infusion every 6 months (600 mg) or Rebif 44 mcg three times a week. Ocrelizumab significantly reduced relapse rates, new lesion formation on MRI and disability progression during the two years of the study compared to Rebif. Amazingly, serous adverse events associated with Ocrelizumab were not more common than serious adverse events with Rebif, a drug considered very safe and a first line therapy for MS. The most common adverse events with Ocrelizumab were infusion related reactions, which can be controlled with premedication. A phase III study of Ocrelizumab in primary progressive MS is still underway.
Why is this important? 1. Many people with MS who have benefited from Rituximab in the past or could benefit from it now are no longer able to receive it because of insurance restrictions. These study results open the way for FDA approval of this class of medication 2. Only one other MS medication (Lemtrada) has shown significant benefits on relapse rates, disability progression and MRI activity over 2 years when compared to a highly active first line DMT (Rebif). Remember, it is relatively easy to be better than placebo treatment. Not only is Ocrelizumab better than Rebif, it is relatively safe and well tolerated over 2 years of treatment. A full understanding of the relative benefits and risks of Ocrelizumab awaits final release of the actual study data for us to review, but the statements in their press release are very exciting. 3. Any treatment that can be administered as infrequently as every 6 months will be welcome to most MS patients 4. Depending on our review of the study data, this drug may benefit certain secondary progressive MS patients All are cause for celebration. Revere (Rip) Kinkel MD Director of the Multiple Sclerosis Program University of California San Diego I recently received a question from a health care journey follower with progressive Multiple Sclerosis asking me a profound question. To paraphrase here is essentially what she asked:
I am a 55 year old woman with progressive MS. I’ve tried numerous disease modifying drugs and either not tolerated them or not benefited from them. I eat well, I exercise regularly and I try to remain positive but I don’t know what else to do. Please provide some advice on what I should do. Until yesterday I did not know how to answer this question without the usual platitudes. Perhaps I was just feeling down; after all, it is easy to let this disease bring you down when you see people every day wanting answers to questions for which there are none, as of yet. But then I received a copy of Louisa Kasdon’s recent article in the Boston Globe about her trip to China with her husband Michael. Follow the link below to the article and once your read it, you will know what to do. Michael, who is confined to a scooter with primary progressive MS, used to be a patient of mine in Boston. Louisa and Michael are brilliant, witty, resourceful and lucky to have the means to live well. But these characteristics do not define their success with life or with MS by any means. Despite their own setbacks and tragedies - and we all experience them in life- Michael and Louisa are defined by their dedication to living, savoring every day and every experience. By living within the moment and savoring those moments and relationships, they maintain an optimism and a hopefulness about life that is an inspiration to us all. When Michael was under my care, a typical visit to the clinic usually began with both Louisa and Michael querying me about my family, my health and my happiness, often ending with an invitation to dinner, which I always accepted given Louisa’s exceptionable abilities as a chef and hostess. It was only after they were satisfied that all was well with their doctor that we could proceed with a discussion of Michael’s MS related problems. Following my evaluation, Michael, who is a physicist and engineer, would provide suggestions on inventions or gadgets that could benefit people with physical limitations. I can almost imagine Louisa and Michael inviting the four star general in their story to lunch so they could further discuss ways to help open the exposition they were visiting to more disabled visitors. So read it and try to emulate Michael and Louisa, as I try every day. http://www.bostonglobe.com/lifestyle/travel/2015/04/18/going-global/EsZ3ublT4M3xsk5yYDhTuL/story.html?event=event25 |
DISCLAIMER:
The medical information and opinions on this site are provided as an information resource only, and are not to be used or relied on for any diagnostic or treatment purposes. The information and opinions expressed do not create any patient-physician relationship, and should not be used as a substitute for professional diagnosis and treatment. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. Archives
January 2020
Categories
All
|
||||||
- Home
- About Us
- Virtual MS Center
- News & Resources
- Seminar Registration
- Health & Wellness
- Blogs
- About MS
-
Symptoms
- Balance and Walking Issues
- Breathing/Respiratory
- Bowel Dysfunction
- Cognitive Dysfunction
- Crying/Laughing Uncontrollably (PBA)
- Depression and Anxiety
- Dizziness/Vertigo
- Dysphagia
- Fatigue
- Foot Drop
- Hearing or Smell or Taste Changes
- Heat Sensitivity
- Leg Weakness
- Loss of Hand Dexterity and Coordination
- Memory and Mutliple Sclerosis
- Migraines
- Numbness/Tingling/Altered Sensation
- Nystagmus and Oscillopsia
- Pain
- Sexual Dysfunction
- Sleep Issues
- Spasticity/Spasms/Cramps
- Speech/Swallowing
- Urination/Bowel Problems
- Vision
- MS Clinics
- MS Topics
- Register With Us
- Terms of Use/Privacy/HIPAA
- MS HealthCare Journey

 RSS Feed
RSS Feed
