 There has been a lot of chatter and excitement this month about the potential benefits of high dose statin therapy in Secondary Progressive MS. Jeremy Chataway and colleagues reported this month in the online edition of Lancet (March 2014) that high dose statin therapy (Simvastatin 80 mg orally once a day) reduced the rate of whole brain atrophy by 43% over 2 years with benefits observed during both the first and second year of the study. Brain Atrophy was chosen as the primary outcome in this study. This means that changes in the size or volume of the entire brain as measured on MRI scans at yearly intervals were used to determine if there was a difference between the 70 patients treated with Simvastatin and the 70 patients treated with placebo. This outcome was justified based on prior studies showing a relationship between the development of brain atrophy and the development of worsening disability in MS patients. By using brain atrophy as the primary outcome the study investigators were able to observe a treatment effect of simvastatin with fewer study patients and a shorter study time interval. In contrast a study to determine if simvastatin reduced the development of sustained disability in MS would require many hundreds of patients potentially for a longer interval. Interestingly, the investigators did report a possible benefit on two clinical disability measures at 2 years, though the significance of this benefit is unclear at this time. These are very hopeful preliminary results but caution must be advised until further studies are completed for the following reasons:
So what should you do with these results if you have secondary progressive MS? After all, the simvastatin was well tolerated in this group of patients. I suggest talking it over with your physician and certainly consider this drug and dosing schedule if you require statin therapy to lower cholesterol or if you have other accepted cardiovascular or cerebrovascular risk factors known to be benefited by statin therapy. I would also encourage you to participate in any Phase III trials of statin therapy that you may hear about in the coming months This is a question I get several times a week and my answer has changed over time with the availability of newer therapies and more information on identifying individual risk factors for the development of Progressive Multifocal Leukoencephalopathy (PML), the complication of treatment with this highly effective drug that we are all trying to avoid. [If you do not know much about Tysabri or PML, I recommend you search our site for a refresher course and then return to this blog]. To better understand if Tysabri therapy is good for you, it is informative to take a short walk down memory lane. When Tysabri first returned to the market place in 2006 and MS specialists started treating patients under the TOUCH program, the risk of PML was estimated at 1/1000. Very quickly we learned about additional risks factors for PML; these new risk factors included,
This was followed by the development of a blood test to determine if your body was making antibodies against the virus that is associated with the development of PML, called the JC virus. Initially, results from the this blood test were reported as either positive or negative; those with a positive test (56% of people) seemed to have almost all the risk of developing PM, whereas those who were negative on this test (44%) had a negligible risk of developing PML. To make matters slightly more complicated, those who were negative on the JC virus antibody test could become positive later (about a 2% yearly conversion rate over the short term). The initial testing showed that about 40% of people with MS were negative on this test and 60% positive. For many neurologist and patients who were risk adverse, this eliminated Tysabri as a treatment option in at least 60% of patients who they felt were good candidates by other criteria. Around this time other highly effective therapies were becoming available for people with MS with their own set of risk factors, particularly Fingolimod (Gilenya) with its risk of cardiac complications, macular edema and reactivation of herpetic infections like shingles. We now have Tecfidera that appears relatively safe and effective, but associated with some unpleasant GI side effects in about 20% of people at the start of treatment. Thankfully, we have further refined the risk stratification for people wishing to consider Tysabri therapy and the FDA recently approved the use of Tysabri as a first line agent (people with no prior disease modifying therapy) in well selected relapsing MS patients. The main change in our risk stratification is based on a free test that measures the actual amount of antibodies your body makes against the JC virus, called the JC Virus antibody index. We no longer consider patients as simply positive or negative for JC antibodies. This new information on stratifying risk by JC virus index in addition to the prior known risk factors, which include duration of therapy and prior immunosuppressant use, forms the basis of assessing your risk of developing PML on Tysabri therapy. More importantly, the new risk stratification scheme reclassifies approximately 25% of the patients previously considered JC virus antibody positive as a relatively lower risk group who may now consider Tysabri therapy. Please see the attached figure to see the entire stratification scheme. Once you know your JC Virus index value (which you can get from your doctor) you can determine whether it is too risky for you to consider Tysabri by simply looking at the chart. First some highlights:
So how do I make these judgments? First, I think it is reasonable to consider an MS therapy as first line in almost all patients if a severe risk of that therapy occurs in less than 1 in 10,000 people. I would consider the therapy first line in selected patients with significant MS disease activity if the risk of PML were less than 1 in 2500 (see table. This would mean a JC virus index value of < 0.9). This is mostly because we have other therapies available that may work and have a lower risk profile. For higher risks of PML, I am inclined to consider therapy only as second or third line but will still use Tysabri in selected cases. So what is your risk profile? What are your thoughts on the risks associated with either Tysabri or any other MS disease modifying therapy? -- Dr. Kinkel PLEASE NOTE: The medical information on this site is provided as an information resource only, and is not to be used or relied on for any diagnostic or treatment purposes. The information and opinions provided do not create any patient-physician relationship, and should not be used as a substitute for professional diagnosis and treatment. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. It seems fitting that on Valentine’s Day I should write about new hope for an MS treatment that may even benefit those with progressive disease! Better yet, this treatment may even target the cause of the disease instead of simply suppressing inflammatory responses within the central nervous system.
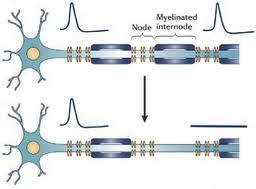
Researchers in Australia have published a case report documenting the rather dramatic response of a severe progressive MS patient to immunotherapy which was designed to boost a specific type of immune response against B Cells latently infected with Epstein Barr Virus, the cause of mononucleosis and a possible cause of MS. The patient experienced: · A dramatic reduction in inflammatory MRI lesions · Improvement in symptoms and neurological findings · A reduction in the production of immunoglobulins within the spinal fluid The reduction in immunoglobulin being produced within the nervous system and measured in spinal fluid was perhaps the most remarkable finding, as it suggests that this treatment eliminated intrathecal (meaning within the spinal fluid) B cells latently infected with Epstein Barr Virus. There is a wealth of direct and indirect evidence that Epstein Barr Virus plays an important role in causing MS. Pathological studies reveal nodules of inflammatory cells scattered across the surface of the brain, often in clusters resembling tiny lymph nodes. Many of these cells are a type of lymphocyte, called a B cell, that are infected with Epstein Barr virus. These inflammatory cells appear to directly damage the underlying cortex of the brain, which may trigger an inflammatory response that spreads throughout the nervous system over many years. It is these same B cells that mature to become the type of cells (called plasma cells) that produce the immunoglobulins in the spinal fluid (CSF) that we use to help diagnose MS. We call these immunoglobulins in the CSF oligoclonal bands. The therapy given to this patient was designed to boost the cytotoxic immune response against EBV infected B Cells. The immune system depends on cytoxic or CD8+ T immune cells to destroy cells infected with viruses, but this cytotoxic response is often lacking in MS patients. They used a novel approach to stimulate a sample of the patient’s blood with AdE1-LMPpoly, a recombinant adenovirus vector that boosts cytotoxic CD8+ T cell responses against Epstein Barr virus proteins expressed by infected B Cells, and then re-injected these boosted cells back into the patient. The patient tolerated the treatment well and improved. Clearly, these results require confirmation in well designed and controlled clinical trials, but for the first time we seem to have a path forward to finding an effective therapy for MS that is based on a reasonable hypothesis regarding the cause of the disease. I wouldn’t be surprised to find a therapy like this available within 5 years if confirmed by additional studies. Happy Valentine’s Day --Dr. Kinkel Abnormal Sensations in MS. Why do they occur and what do they mean? Abnormal sensations and pain are a common experience for people with MS. To understand this a little better, I need to begin with a short explanation of how the nervous system works. The images above are cross sectional depictions of two neurons and their associated axon. Neurons, as you can see, are strange looking cells with tentacles (axons and dendrites) that reach out and connect with other neurons in defined circuits. The connections made by these neurons are the means by which your nervous system receives information, interprets and manipulates information and acts on information. This information may come in the form of sights, sounds, tastes, smells, sensations or language. The information flow can also start from spontaneously generated thought and contemplation. The myelin that is damaged in MS is shown in blue (called the myelinated internode in the picture) wrapped around segments of the axon (shown in blue green) with gaps between the myelinated internode that are called the Nodes of Ranvier (labeled Node in the picture). As you may know it is common in medicine to name things after their founder and a French physician in the late 19th and early 20th century named Ranvier discovered this peculiar feature of our nervous systems. The top image is a normal neuron-axon unit and the lower image depicts a neuron-axon unit that has been stripped of myelin in an area but with no permanent damage to the axon. Now why is this a problem?
The difficult question is whether these new sodium channels that sprout all axons are harmful. There is some research that indicates that the effects of these sodium channels may lead to permanent damage to the neuron, but attempts so far to prevent this from occurring by administering drugs that block sodium channels have not been successful. |
DISCLAIMER:
The medical information and opinions on this site are provided as an information resource only, and are not to be used or relied on for any diagnostic or treatment purposes. The information and opinions expressed do not create any patient-physician relationship, and should not be used as a substitute for professional diagnosis and treatment. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. Archives
January 2020
Categories
All
|
- Home
- About Us
- Virtual MS Center
- News & Resources
- Seminar Registration
- Health & Wellness
- Blogs
- About MS
-
Symptoms
- Balance and Walking Issues
- Breathing/Respiratory
- Bowel Dysfunction
- Cognitive Dysfunction
- Crying/Laughing Uncontrollably (PBA)
- Depression and Anxiety
- Dizziness/Vertigo
- Dysphagia
- Fatigue
- Foot Drop
- Hearing or Smell or Taste Changes
- Heat Sensitivity
- Leg Weakness
- Loss of Hand Dexterity and Coordination
- Memory and Mutliple Sclerosis
- Migraines
- Numbness/Tingling/Altered Sensation
- Nystagmus and Oscillopsia
- Pain
- Sexual Dysfunction
- Sleep Issues
- Spasticity/Spasms/Cramps
- Speech/Swallowing
- Urination/Bowel Problems
- Vision
- MS Clinics
- MS Topics
- Register With Us
- Terms of Use/Privacy/HIPAA
- MS HealthCare Journey

 RSS Feed
RSS Feed
